Wed, May 14, 2025
[Archive]
Abstract: (1095 Views)
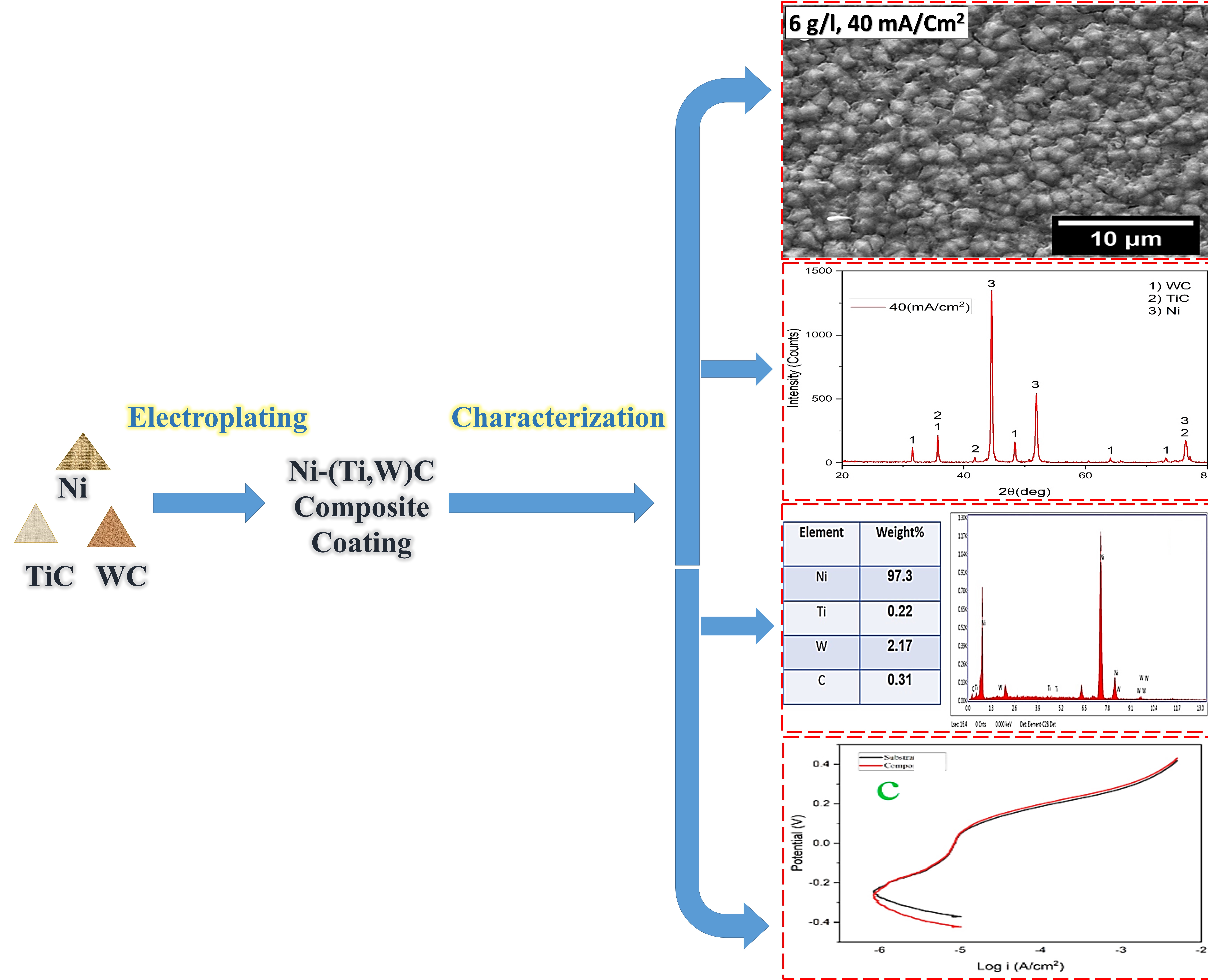
The aim of this study was to investigate the effect of current density on the microstructure of electrodeposited Ni–WC–TiC composite coatings on 304 stainless steel and compare the corrosion resistance of the coating and substrate in a 3.5 wt.% sodium chloride solution. A Watts nickel bath was employed under direct current (DC) conditions. Microstructure, elemental composition, and phase composition analyses were conducted using scanning electron microscopy (SEM) equipped with energy-dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD), respectively. The results revealed that the coating formed at a current density of 40 mA/cm² exhibited a denser microstructure with higher cohesion and uniformity compared to coatings produced at other current densities. The corrosion resistance of the coating and substrate was evaluated using Tafel and electrochemical impedance spectroscopy (EIS) analyses. The corrosion test results indicated that the substrate exhibited superior corrosion resistance compared to the coating. Based on the dynamic polarization test plots, the corrosion mechanism of the substrate is active-quasi passive, with a pseudo-passive layer forming on the sample which remains stable within the potential range of -0.17 to 0.17 V. Beyond this potential range, the sample becomes susceptible to pitting. In the coated sample, the corrosion behavior is similar to that of the substrate, with the exception that the pseudo-passive layer remains stable within a narrower potential range of -0.19 to 0.08 V.
Type of Study: Research Paper |
Subject:
Synthesis and preparation of materials to meet the requirements of AM techniques
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |